THE HISTORY OF THE ATOMIC MODEL
Kalam 6SE
Scroll down
The model of the atom has changed massively (for something that has so little mass) over the centuries.
Like many things in science, models are used to help us understand how things interact with each other, and can allow scientists to predict the nature of things with high accuracy.
EARLY
IDEAS

The theory of the atomic model goes all the way back to the Ancient Greeks and can be traced to Leucippus and his pupil, Democritus. The word atom comes from the Greek word "atomos", which means uncuttable. At that point of time, it was thought that atoms were the most fundamental kind of matter around, it could not be broken down any further.
PLUM
PUDDING
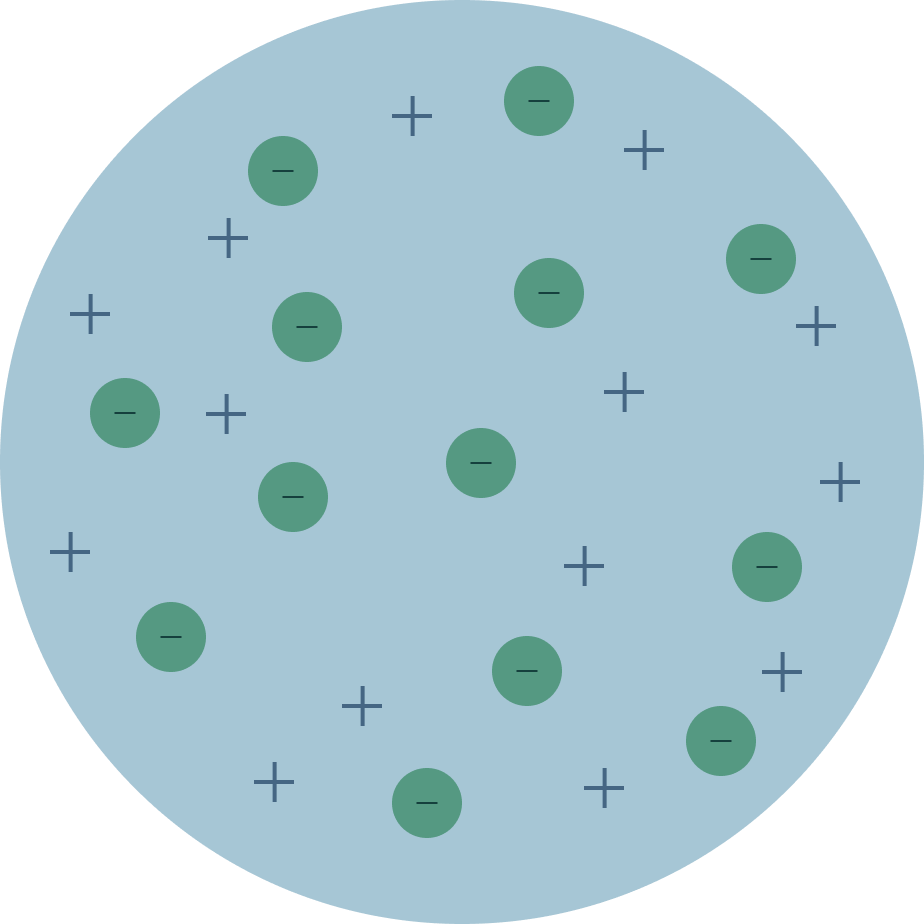
J.J Thomson had discovered that atoms weren't indvisiable as the Greeks had once thought. After carrying out an experiment, he found out that cathode rays were attracted by positively charged metal plates but repelled by negatively charged ones. Since like charges repel each other, Thomon deduced the rays were negatively charged
He deduced the mass of these particles were 2000x lighter than hydrogen. Originally called the "corpuscle", Thomson had discovered what we now know as electrons, proving the atom was composed of smaller parts.
Thomson then suggested the plum pudding model, a "pudding" of positive matter had randomly distributed negatively charged "plums". Ions form when the negative "plums" (electrons) were knocked out or into the atom.
This model was an improvement over the old greek model as the idea of it being split into positive and negative matter can explain things like ionic bonding.
GOLD FOIL
EXPERIMENT
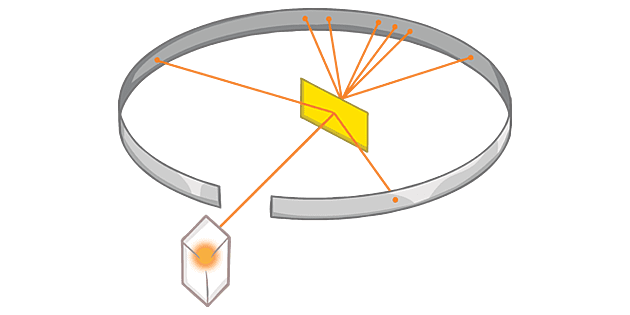
Ernest Rutherford, a scientist from New Zealand who investigated the chemistry of radioactive substances, decided to use the alpha particles emitted by sources to research the atom.
Whilst Rutherford orchestrated the experiment, it was actually his students, Geiger and Marsden who actually ran the experiment.
The apparatus used included :
- Alpha particle source
- Gold foil (very thin)
- A vacuum chamber
- Flourescent screen
- Detector
The alpha particle source was set up in such a way that alpha particles only travel towards the gold foil. The gold foil was inside of a vacuum chamber to reduce the chances that anything interferes with the results. A flourescent screen was placed around the vacuum chamber, which would cause it to glow in spots when an alpha particle collides.
The observed results showed that most of the alpha particles passed through the gold without deflections, but a few were deflected and very few bounced off the foil. This was measured by observing points around the flourescent screen glowing when the alpha particles came in contact.
Rutherford said that this was as if a 15in shell fired at a tissue paper bounced back.
Since the vast majority of the particles went through without being deflected, the idea the atom was uniform did not hold, else the majority would be deflected. Furthermore, since some were being completely deflected, it must mean that they had hit something. It must have a high mass (in proportion to the whole atom) and a high charge. Because the chances of hitting this "nucleus" with randomly emitted alpha particles was low, it must be much much smaller than the atom itself
RUTHERFORD
SCATTERING RESULTS
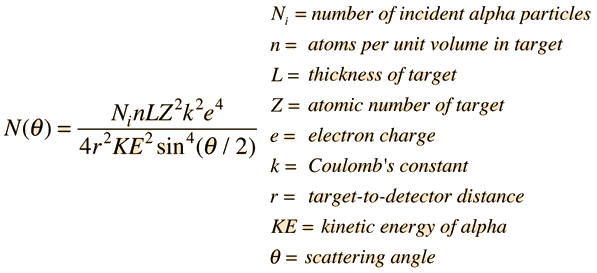
Rutherford derived the equation pictured after assuming that: most of the atom is empty space; the centre of the atom holds all the mass and all of the positive charge; alpha particles scatter because of the electrostatic force between the alpha particle and the nucleus. The equation also shows that the closer an alpha particle gets to the nucleus, the more it is deflected away. Furthermore, since the number of alpha particles changed depending on the element they were being fired at, different elements must have different amounts of charges. This new model showed the difference elements have with one another, with different elements having different proton numbers.
NIELS BOHR'S
WORK

Rutherford's model did not explain why electrons orbitted the atom rather than spiral down into it due to the electrostatic force. The Danish physicist, Niels Bohr, was trying to solve the problems found within Rutherford's model and created a new model.
Bohr's model suggested the idea of electrons being at different energy levels and electrons can only be at these specific energy levels. Electrons can move up and down these energy levels by absorbing and releasing energy. This was good, but it didn't solve all the problems of atomical models.
SCHRÖDINGER'S
CLOUD

Austrian physicist, Erwin Schrödinger (yes the same guy behind the cat in the box), proposed that instead of moving in fixed orbits or shells, electrons behave like waves. This was possible due to the wave-particle duality of electrons, similar to light. His model shows the nucleus being surrounded by clouds of electron density, the clouds representing the probability of finding electrons in the given volume of space. These clouds are known as electron orbitals and is the accepted model today.
CHADWICK'S
NEUTRON

There still was an issue with the atomic model at the time, a helium nucleus would have an atomic number of 2, but a mass number of 4 - it seemed like there was something missing. Rutherford had previously proposed that a proton and an electron could combine to make a neutral particle, but it was dismissed as the neutral particle would be hard to detect and there was no real evidence.
Chadwick noticed some odd unexpected behaviour concerning radiation after Frédéric and Irène Joliot-Curie observed an unidentified piece of radiation from beryllium as it hit a paraffin wax target. Chadwick tried similar experminets himself and became convinced that the radiation was a neutral particle about the same mass as a proton. Since this particle had no charge, Chadwick observed they penetrated deeper into a target than protons. Chadwick had discovered the neutron.
From being the "uncuttable" particles called "atomos" by the Greek, we now know that atoms are actually composed up of electrons, protons and neutrons. Today we also know that those sub atomic particles can be split down even further into quarks and antiquarks and as new scientific experiments and data come to light, who knows how much more further the current atomic model will be refined by?